Catheter-Associated Urinary Tract Infections & Sepsis
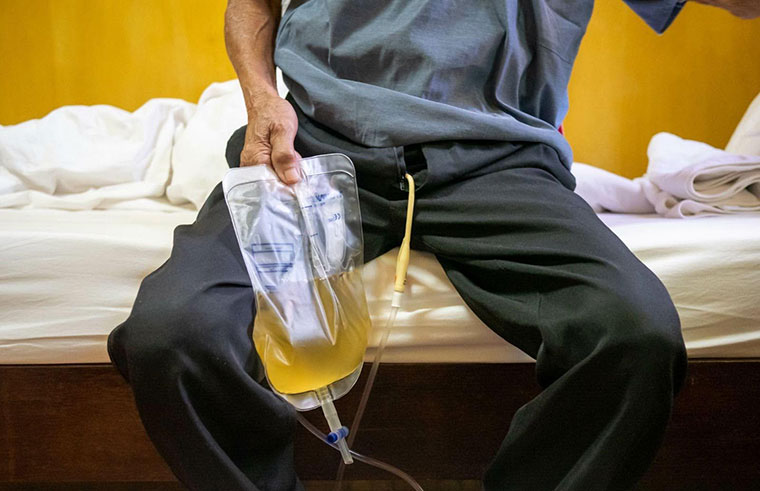
Catheter-associated urinary tract infections (CAUTIs) are among the most prevalent healthcare-associated infections, accounting for up to 40% of hospital-acquired infections.
Introduction
These infections occur when bacteria enter the urinary tract via an indwelling catheter, leading to complications such as increased morbidity, prolonged hospital stays, and rising healthcare costs. CAUTIs can be classified into two types: symptomatic infections with urinary tract manifestations and catheter-associated asymptomatic bacteriuria (CA-ASB), where bacteria are present without overt symptoms. Proper differentiation between these conditions is essential to avoid unnecessary antibiotic use, which contributes to antimicrobial resistance.
Hospitalized male patients with disabilities, particularly those using wheelchairs, are at heightened risk for CAUTIs due to factors such as prolonged catheterization, reduced mobility, and underlying comorbidities. The presence of an indwelling catheter comprises the urinary tract’s natural defense mechanisms, promoting bacterial colonization and biofilm formation. Given the increasing burden of antibiotic-resistant infections, effective prevention strategies, improved catheter materials, and strict adherence to catheter management protocols are crucial in reducing CAUTI rates and improving patient outcomes.
Epidemiology and Risk Factors
Epidemiology and Risk Factors of CAUTI in America
Catheter-associated urinary tract infections (CAUTIs) are among the most prevalent healthcare-associated infections (HAIs). In North and South America, the incidence of CAUTI is estimated at 5.70 cases per 1,000 catheter days, a figure that underscores the significant burden of these infections in healthcare settings. Patients in intensive care units (ICUs) are particularly vulnerable, as CAUTIs contribute to higher mortality rates and complications that can prolong hospital stays.
Risk Factors for CAUTI
-
Prolonged Catheterization – CAUTI risk rises after 6 days; nearly all develop bacteriuria by day 30. Non-surgical placement and urine monitoring increase infection risk.
-
Limited Mobility & Disability – Neurological disorders, spinal injuries, and immobility cause urine stasis, fostering infection. Bedridden patients are highly vulnerable.
-
Gender Differences – Males face higher CAUTI risk due to prolonged catheterization. Malnutrition and renal issues increase susceptibility.
-
Advanced Age – Patients over 70 have higher CAUTI prevalence due to chronic conditions, weak immunity, and comorbidities.
-
Diabetes Mellitus – Neuropathy causes poor bladder emptying, while glucosuria promotes bacterial growth.
-
Hygiene & Incontinence – Poor perineal hygiene and fecal incontinence encourage bacterial colonization and infection.
-
Catheter Type & Care – Latex catheters harbor more bacteria than silicone. Aseptic technique errors raise infection risk.
Pathophysiology of CAUTI Leading to Sepsis
Catheter-associated urinary tract infections (CAUTIs) develop when bacteria from the rectal or periurethral area travel up the urinary catheter and enter the bladder. Normally, urination helps flush out bacteria, but a catheter provides a direct pathway, bypassing the body’s natural defenses. Once inside the bladder, bacteria such as Escherichia coli, Pseudomonas aeruginosa, and Proteus mirabilis attach to the catheter surface and start forming biofilms—protective layers that make the bacteria harder to eliminate. Biofilm formation can begin within minutes, allowing bacteria to multiply and persist despite the body’s immune response or antibiotic treatment.
As the infection progresses, bacteria use pili and adhesins to stick to the bladder lining, triggering inflammation. The immune system responds by sending neutrophils to fight the infection, but the biofilm structure makes it difficult to clear. Over time, bacterial toxins and enzymes damage bladder tissues, creating small openings that allow bacteria to enter the bloodstream. Once in circulation, they can spread throughout the body, leading to bacteremia (bacteria in the blood) and, in severe cases, sepsis. Sepsis is a life-threatening condition where the body’s response to infection causes widespread inflammation, leading to organ damage and failure.
Clinical Manifestations
Catheter-associated urinary tract infections (CAUTIs) can present with a broad range of symptoms, which may be subtle in certain populations. Common symptoms include fever, suprapubic discomfort, flank pain, new-onset or worsening urinary urgency, frequency, dysuria, and cloudy or foul-smelling urine. In patients with neurogenic bladder or other disabilities, symptoms may manifest differently, including increased spasticity, autonomic dysreflexia, malaise, or a general sense of unease. Mental status changes, including confusion or lethargy, are particularly concerning in older or cognitively impaired patients and may be the first indication of infection.
Progression to sepsis is a critical concern in CAUTI. Signs indicating escalation include persistent high fever, tachycardia, hypotension, respiratory distress, and altered mental status. Laboratory markers such as elevated white blood cell count, lactate levels, and procalcitonin can indicate systemic involvement. Early recognition and intervention are essential to prevent severe complications.

Diagnostic Approaches
Diagnosing CAUTI involves clinical evaluation and laboratory tests. Standard diagnostic criteria include the presence of symptoms consistent with UTI and a positive urine culture with bacterial growth exceeding 10³ CFU/mL from a catheterized specimen. However, controversy exists regarding the optimal threshold, with some guidelines recommending 10⁵ CFU/mL. Urine culture interpretation should consider prior antibiotic exposure, symptom presentation, and patient-specific factors.
Challenges arise in diagnosing CAUTI in patients with disabilities, as traditional symptoms may be absent or masked by underlying conditions. Neurogenic bladder patients may not exhibit typical urinary symptoms, making diagnosis reliant on alternative signs such as autonomic dysreflexia or increased spasticity. Additionally, distinguishing CAUTI from asymptomatic bacteriuria is challenging, leading to potential overdiagnosis and unnecessary antibiotic use. Urinalysis markers like pyuria and leukocyte esterase lack specificity, as they may be present in both CAUTI and asymptomatic bacteriuria. Biomarkers such as IL-6, IL-8, and NGAL show promise in improving diagnostic accuracy, but further research is needed for widespread clinical implementation. Ultimately, an individualized approach, incorporating symptom assessment, laboratory findings, and clinical judgment, remains key in diagnosing CAUTI and preventing complications like sepsis.
Preventive Strategies
Best Practices for Catheter Use and Maintenance to Prevent Infections
Preventing catheter-associated urinary tract infections (CAUTI) is crucial, especially for wheelchair-bound male patients who often require long-term catheterization. The most effective strategy is minimizing catheter use and removing it as soon as possible. Studies show that each day a catheter remains in place, the risk of developing bacteriuria increases by 3–7%, with a 0.3% daily risk of UTI. Therefore, daily evaluation of catheter necessity is essential.
Alternative methods such as clean intermittent catheterization (CIC) or external catheters (e.g., condom catheters) should be considered when possible. The CDC recommends external catheters as a safer option to reduce infection risk. Additionally, aseptic insertion techniques, proper hand hygiene, and closed drainage systems are critical. The catheter should be securely fastened to prevent movement and minimize urethral trauma. Keeping the drainage bag below bladder level helps prevent backflow, reducing infection risk.
Hospitals implementing electronic reminders for catheter removal and nurse-led protocols have seen a 37% reduction in CAUTI rates. Similarly, a U.S.-based initiative using catheter removal reminders, ultrasound bladder scanners, and proper insertion techniques led to a significant decrease in CAUTI rates from 2.40 to 2.05 per catheter day.
Role of Healthcare Providers in Minimizing CAUTI Risk
Healthcare providers play a key role in CAUTI prevention. Proper training on aseptic techniques and securement methods reduces infection rates. Using antimicrobial-coated catheters has mixed results, but hydrogel-coated options may reduce bacterial adherence and irritation. Regular staff education on best practices and maintaining sufficient medical supplies are essential to preventing infections. Additionally, protocols for timely catheter replacement—typically every four weeks or sooner in cases of obstruction or infection—are crucial in reducing bacterial buildup and biofilm formation.
Antibiotic prophylaxis for long-term indwelling catheters remains debated due to limited evidence. Studies suggest short-term benefits, particularly in post-surgical patients. A 2005 Cochrane review indicated weak support for reducing CAUTI, while a 2013 meta-analysis showed a 5.8% risk reduction at catheter removal. Ciprofloxacin, commonly used post-prostatectomy, showed no significant CAUTI reduction. Despite risks of antimicrobial resistance, many urologists still prescribe antibiotics.
Alternative preventive strategies include D-mannose, which inhibits bacterial adhesion in the urinary tract, and probiotics, which help maintain a healthy microbiome and reduce UTI recurrence. While some studies support their use, further research is needed.
Treatment Strategies for CAUTI
Effective management of CAUTIs involves a combination of catheter removal or exchange and targeted antibiotic therapy. If a catheter is no longer needed, it should be removed; otherwise, it should be replaced before initiating antibiotics, especially if it has been in place for over two weeks. A clinical trial demonstrated that catheter replacement prior to antibiotic treatment resulted in faster symptom resolution and lower recurrence rates.
Antibiotic selection should be guided by illness severity, local resistance patterns, and individual patient factors. Empiric choices should avoid amoxicillin, TMP-SMX, and amoxicillin/clavulanic acid due to high resistance rates. Ciprofloxacin is reserved for select cases where resistance is low and no safer alternatives exist.
Treatment duration varies based on response; a seven-day course is typically sufficient for those with prompt symptom resolution, while a longer regimen may be required for more severe cases.
Conclusion
Preventing CAUTI in male patients with urinary incontinence requires a comprehensive approach, including minimizing catheter use, maintaining hygiene, and using alternative solutions when possible. Absorbent wraps offer a practical option to manage incontinence while reducing catheter dependence, thereby lowering infection risks and improving patient comfort and quality of life.
For those seeking support, we invite you to purchase a 10 Count Trial Pack here or request a professional-use sample pack for healthcare institutions here.
References:
- Al-Hazmi, H. (2015). Role of duration of catheterization and length of hospital stay on the rate of catheter-related hospital-acquired urinary tract infections. Research Reports in Urology, 7, 41-47. https://doi.org/10.2147/RRU.S75419
- Bonkat, G., Bartoletti, R., Bruyère, F., Cai, T., Geerlings, S. E., Köves, B., Kranz, J., Schubert, S., Pilatz, A., Veeratterapillay, R., Wagenlehner, F. M. E., Bausch, K., Devlies, W., Horváth, J., Leitner, L., Mantica, G., Mezei, T., Smith, E. J., & Ali, H. (2024). EAU guidelines on urological infections (Limited text update April 2024). European Association of Urology.
- Chuang, L., & Tambyah, P. A. (2021). Catheter-associated urinary tract infection. Journal of Infection and Chemotherapy, 27(10), 1400-1406. https://doi.org/10.1016/j.jiac.2021.07.022
- Flores-Mireles, A. L., Walker, J. N., Caparon, M., & Hultgren, S. J. (2015). Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nature Reviews Microbiology, 13(5), 269-284. https://doi.org/10.1038/nrmicro3432
- Foxman, B., Cronenwett, A. E., Spino, C., Berger, M. B., & Morgan, D. M. (2015). Cranberry juice capsules and urinary tract infection after surgery: Results of a randomized trial. American Journal of Obstetrics and Gynecology, 213(2), 194.e1-8. https://doi.org/10.1016/j.ajog.2015.04.003
- Girard, R., Gaujard, S., Pergay, V., Pornon, P., Martin-Gaujard, G., & Bourguignon, L. (2017). Risk factors for urinary tract infections in geriatric hospitals. Journal of Hospital Infection, 97(1), 74-78. https://doi.org/10.1016/j.jhin.2017.05.007
- Gould, C. V., Umscheid, C. A., Agarwal, R. K., Kuntz, G., & Pegues, D. A. (2010). Guideline for prevention of catheter-associated urinary tract infections 2009. Infection Control and Hospital Epidemiology, 31(4), 319-326. https://doi.org/10.1086/651091
- Hooton, T. M., Bradley, S. F., Cardenas, D. D., Colgan, R., Geerlings, S. E., Rice, J. C., Saint, S., Schaeffer, A. J., Tambyah, P. A., Tenke, P., & Nicolle, L. E. (2010). Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clinical Infectious Diseases, 50(5), 625-663. https://doi.org/10.1086/650482
- Ji, L., Badalato, G. M., Chung, D. E., et al. (2020). Cranberry products for the prevention of catheter-associated urinary tract infections. Current Bladder Dysfunction Reports, 15, 303-307. https://doi.org/10.1007/s11884-020-00610-3
- Kunin, C. M., & McCormack, R. C. (1966). Prevention of catheter-induced urinary-tract infections by sterile closed drainage. New England Journal of Medicine, 274(21), 1155-1161. https://doi.org/10.1056/NEJM196605262742101
- Lusardi, G., Lipp, A., & Shaw, C. (2013). Antibiotic prophylaxis for short-term catheter bladder drainage in adults. Cochrane Database of Systematic Reviews, 2013(7), CD005428. https://doi.org/10.1002/14651858.CD005428.pub2
- Nanda, N., & Juthani-Mehta, M. (2009). Novel biomarkers for the diagnosis of urinary tract infection-a systematic review. Biomarker Insights, 4, 111-121. https://doi.org/10.4137/bmi.s3155
- Nicolle, L. E. (2014). Catheter-associated urinary tract infections. Antimicrobial Resistance and Infection Control, 3, 23. https://doi.org/10.1186/2047-2994-3-23
- Parsons, C. L. (1986). Pathogenesis of urinary tract infections: Bacterial adherence, bladder defense mechanisms. Urologic Clinics of North America, 13(4), 563-568.
- Talja, M., Korpela, A., & Järvi, K. (1990). Comparison of urethral reaction to full silicone, hydrogen-coated, and siliconised latex catheters. British Journal of Urology, 66(6), 652-657. https://doi.org/10.1111/j.1464-410x.1990.tb07203.x
- Tissot, E., Limat, S., Cornette, C., & Capellier, G. (2001). Risk factors for catheter-associated bacteriuria in a medical intensive care unit. European Journal of Clinical Microbiology and Infectious Diseases, 20(4), 260-262. https://doi.org/10.1007/s100960100480
- Trautner, B. W., & Morgan, D. J. (2020). Imprecision medicine: Challenges in diagnosis, treatment, and measuring quality for catheter-associated urinary tract infection. Clinical Infectious Diseases, 71(9), e520-e522. https://doi.org/10.1093/cid/ciaa467
- Venkataraman, R., & Yadav, U. (2023). Catheter-associated urinary tract infection: An overview. Journal of Basic and Clinical Physiology and Pharmacology, 34(1), 5-10. https://doi.org/10.1515/jbcpp-2022-0152
- Werneburg, G. T. (2022). Catheter-associated urinary tract infections: Current challenges and future prospects. Research Reports in Urology, 14, 109-133. https://doi.org/10.2147/RRU.S273663


