Understanding Mybertriq: A Comprehensive Look at its Role in Treating Bladder Spasms
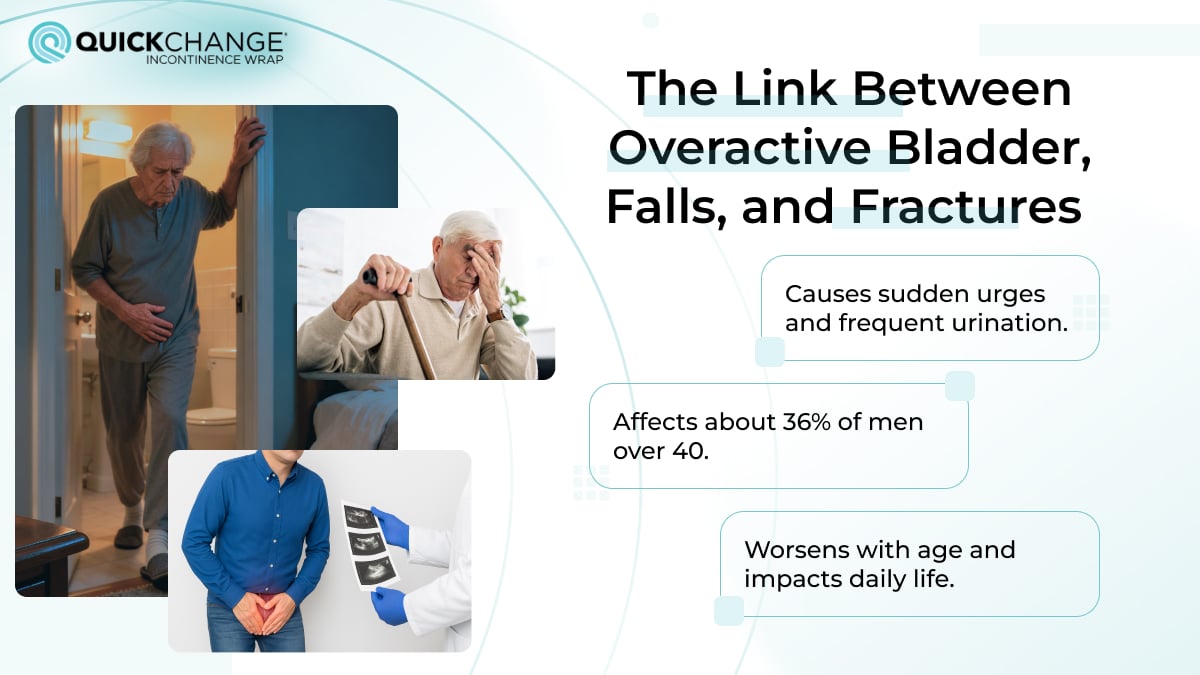
Overactive bladder syndrome (OAB) is a chronic, disabling condition affecting millions worldwide. It is marked by a range of symptoms, including frequent bladder spasms, sudden urges to urinate, and sometimes urge incontinence. These symptoms can significantly impact quality of life—not only physically, but also psychologically and socially. For many, the condition disrupts daily activities, causing distress and inconvenience.
OAB and bladder spasms vary from person to person, making diagnosis and treatment challenging. Patients with OAB often experience frustration due to the unpredictable nature of their symptoms, which can also be difficult for medical teams to address effectively. This condition requires careful diagnosis, clear explanations, and the full support of healthcare providers, as understanding and collaboration are essential for managing symptoms.
Managing OAB often involves a progressive approach, including lifestyle changes, behavioral strategies, and, if needed, medications. While traditional drugs can be effective, they often have side effects, leading to the development of newer, bladder-targeted options like Mirabegron. The main goal in treating OAB is to improve quality of life, providing patients with individualized care and symptom relief that helps restore comfort and control.
Understanding Mirabegron
Mirabegron (Mybertiq) is a medication specifically designed to help relieve bladder spasms and manage symptoms of overactive bladder (OAB). It works as a β3-adrenoceptor agonist, meaning it targets specific receptors in the bladder muscle to help it relax. By doing so, Mirabegron reduces the urgency and frequency of urination and can also lessen episodes of incontinence.
Mirabegron has been studied extensively, with research showing its effectiveness in improving bladder control and enhancing quality of life for patients with OAB. Clinical trials have shown that over 12 weeks, it significantly reduces the number of times patients need to urinate, enhances bladder control, and improves quality of life. Compared to other drugs for OAB, such as antimuscarinics, Mirabegron is often preferred due to its lower risk of side effects like dry mouth. This makes it an appealing option, especially for patients who have experienced issues with antimuscarinic treatments. Overall, Mirabegron provides a well-tolerated and effective alternative for managing OAB symptoms, allowing patients to regain control over their daily lives.
Uses and Effectiveness
Mirabegron has been proven effective in relieving symptoms of overactive bladder (OAB) and bladder spasms in multiple large studies. In the BLOSSOM trial, patients taking Mirabegron (100 mg or 150 mg twice daily) experienced a notable reduction in the frequency of urination compared to those given a placebo. Patients taking Mirabegron reported nearly twice the improvement in reducing bathroom trips per day compared to the placebo group. Importantly, the rate of side effects for Mirabegron was similar to that for the placebo, which supports its tolerability.
Other trials, including the DRAGON and SCORPIO studies, further demonstrated Mirabegron’s benefits for reducing incontinence and urgency episodes. The SCORPIO study, for example, compared Mirabegron with both placebo and tolterodine (another OAB medication) over 12 weeks and found that Mirabegron significantly decreased daily episodes of incontinence and frequent urination. Notably, patients who were new to antimuscarinic therapy or had used it previously both showed similar improvements, suggesting that Mirabegron works well for a broad range of patients.
Case Reports and Patient Experiences
Patient-reported outcomes (PROs) offer insights into how Mirabegron helps people with overactive bladder (OAB) manage their symptoms and improve daily life. In the SCORPIO trial, a 12-week study involving patients with OAB, those taking Mirabegron (50 mg) reported meaningful improvements in their quality of life (QoL), including feeling more in control of their condition. Patients on Mirabegron were also able to cope better with the disease and had fewer concerns about it, while experiencing improved productivity and reduced work absences compared to those on placebo or tolterodine, a common OAB medication.
Another study, PERSPECTIVE, looked at patient-reported outcomes in real-world settings and found that both Mirabegron and antimuscarinic therapies (another treatment for OAB) led to similar improvements in symptom scores and quality of life. This evidence supports Mirabegron’s role as a practical option with benefits that parallel those of existing OAB treatments.
A pooled analysis of three phase III trials further highlighted that patients taking Mirabegron (50 mg) experienced significant reductions in the frequency and severity of bothersome symptoms like urgency and night waking. The study noted improvements in patients’ perception of their bladder condition and their ability to cope with symptoms, showing that Mirabegron has a positive and sustained impact on daily life for people managing OAB.
Potential Side Effects and Precautions
While Mirabegron is generally well tolerated, patients should be aware of potential side effects and discuss any concerns with their healthcare provider. Common side effects include increased blood pressure, headaches, and mild elevations in heart rate. In studies like SCORPIO and Yamaguchi, adverse events were monitored closely, with blood pressure and heart rate regularly measured throughout the trials. Hypertension, defined as systolic blood pressure above 140 mmHg or diastolic above 90 mmHg on consecutive visits, was noted as a potential side effect, although cases were mild and similar to placebo groups.
Some cardiovascular side effects, such as tachycardia and palpitations, were also recorded but occurred at a similar rate as in the placebo groups. The CAPRICORN study found that treatment-emergent adverse events (TEAEs) in Mirabegron-treated patients were mostly mild or moderate and were generally no more common than in those taking a placebo. A slight increase in pulse rate (around 2 beats per minute) was observed at the four-week mark but did not increase over time, and heart rate returned to normal after treatment stopped.
Long-term studies, such as the TAURUS and CAPRICORN trials, confirm that Mirabegron maintains its effectiveness and is well-tolerated over extended use. The 12-month TAURUS study revealed that adverse events were low, and most patients could continue Mirabegron without issues. Results consistently showed fewer side effects like dry mouth compared to other OAB treatments, and older adults in particular reported better quality of life while on Mirabegron. These findings make Mirabegron a reliable option for long-term management of OAB and bladder spasms, with sustained benefits over time.
Future Directions in Treatment
Research into the treatment of overactive bladder (OAB) continues to evolve, with promising new therapies on the horizon. One key area of focus is the development of additional β3-adrenoceptor agonists, similar to Mirabegron. Solabegron, for example, has shown positive results in phase II trials, suggesting it may become another effective option for OAB treatment. Ritobegron, another β3 agonist, has demonstrated potent effects in animal studies, though human trials are still pending. These medications may offer further alternatives for patients who do not respond to current therapies.
In addition to new β3 agonists, researchers are exploring combination therapies. Combining Mirabegron with other treatments could potentially enhance its effectiveness or reduce side effects, providing a more tailored approach to OAB management. Ongoing clinical trials are examining how Mirabegron might work alongside other medications, like antimuscarinics, to improve patient outcomes.
As new medications and combination therapies are investigated, guidelines for treating OAB may shift. Currently, Mirabegron is recommended as a second-line treatment after anticholinergics, but growing evidence supporting its efficacy, particularly in treatment-naïve patients, may influence future recommendations. Staying updated on these developments can offer hope for more effective and personalized treatment options for those managing OAB.
Conclusion and Key Takeaways
Mirabegron offers a promising solution for managing bladder spasms and overactive bladder (OAB), providing patients with significant improvements in symptom control and quality of life. By targeting the β3-adrenoceptors in the bladder, it reduces the urgency and frequency of urination, making it a well-tolerated option, especially for those who struggle with side effects from other treatments. While Mirabegron has shown positive results, it is important for patients to monitor potential side effects, such as elevated blood pressure, and consult their healthcare providers regularly. With ongoing research into new therapies and combination treatments, there is hope for even better options in the future. Always consult your healthcare provider to find the best treatment plan for you.
In addition to medication, supporting comfort with absorbent products like QuickChange Wrap can further enhance patient well-being.
For those looking to experience the advantages of these wraps, we invite you to purchase a 10 Count Trial Pack here . If you are part of a healthcare institution, please request a professional use sample pack here.
References:
- Carlson KV, Rovner ES, Nair KV, Deal AS, Kristy RM, Schermer CR. Factors Associated with Improvements in Patient-Reported Outcomes During Mirabegron or Antimuscarinic Treatment of Overactive Bladder Syndrome: A Registry Study (PERSPECTIVE). Adv Ther. 2019 Aug;36(8):1906-1921. doi: 10.1007/s12325-019-00994-7. Epub 2019 Jun 20. PMID: 31222714; PMCID: PMC6822866.
- Castro-Diaz D, Chapple CR, Hakimi Z, Blauwet MB, Delgado-Herrera L, Lau W, Mujais S. The effect of mirabegron on patient-related outcomes in patients with overactive bladder: the results of post hoc correlation and responder analyses using pooled data from three randomized Phase III trials. Qual Life Res. 2015 Jul;24(7):1719-27. doi: 10.1007/s11136-014-0904-4. Epub 2015 Feb 17. PMID: 25688038; PMCID: PMC4483243.
- Chapple CR, Amarenco G, López Aramburu MA, Everaert K, Liehne J, Lucas M, Vik V, Ridder A, Snijder R, Yamaguchi O; BLOSSOM Investigator Group. A proof-of-concept study: mirabegron, a new therapy for overactive bladder. Neurourol Urodyn. 2013 Nov;32(8):1116-22. doi: 10.1002/nau.22373. Epub 2013 Feb 19. PMID: 23424164.
- Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T, Dorrepaal C, Martin N. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013 Feb;63(2):296-305. doi: 10.1016/j.eururo.2012.10.048. Epub 2012 Nov 6. PMID: 23195283.
- Khullar V, Amarenco G, Angulo JC, Blauwet MB, Nazir J, Odeyemi IA, Hakimi Z. Patient-reported outcomes with the β3 -adrenoceptor agonist mirabegron in a phase III trial in patients with overactive bladder. Neurourol Urodyn. 2016 Nov;35(8):987-994. doi: 10.1002/nau.22844. Epub 2015 Aug 19. PMID: 26288118.
- Khullar V, Cambronero J, Angulo JC, Wooning M, Blauwet MB, Dorrepaal C, Martin NE. Efficacy of mirabegron in patients with and without prior antimuscarinic therapy for overactive bladder: a post hoc analysis of a randomized European-Australian Phase 3 trial. BMC Urol. 2013 Sep 18;13:45. doi: 10.1186/1471-2490-13-45. PMID: 24047126; PMCID: PMC3849064.
- Maruyama I, Tatemichi S, Goi Y, Maruyama K, Hoyano Y, Yamazaki Y, Kusama H. Effects of ritobegron (KUC-7483), a novel selective β3-adrenoceptor agonist, on bladder function in cynomolgus monkey. J Pharmacol Exp Ther. 2012 Jul;342(1):163-8. doi: 10.1124/jpet.112.191783. Epub 2012 Apr 16. PMID: 22511202.
- O'Kane M, Robinson D, Cardozo L, Wagg A, Abrams P. Mirabegron in the Management of Overactive Bladder Syndrome. Int J Womens Health. 2022 Sep 16;14:1337-1350. doi: 10.2147/IJWH.S372597. PMID: 36147890; PMCID: PMC9487925.
- Ohlstein EH, von Keitz A, Michel MC. A multicenter, double-blind, randomized, placebo-controlled trial of the β3-adrenoceptor agonist solabegron for overactive bladder. Eur Urol. 2012 Nov;62(5):834-40. doi: 10.1016/j.eururo.2012.05.053. Epub 2012 Jun 5. PMID: 22695239.
- Scarneciu I, Lupu S, Bratu OG, Teodorescu A, Maxim LS, Brinza A, Laculiceanu AG, Rotaru RM, Lupu AM, Scarneciu CC. Overactive bladder: A review and update. Exp Ther Med. 2021 Dec;22(6):1444. doi: 10.3892/etm.2021.10879. Epub 2021 Oct 14. PMID: 34721686; PMCID: PMC8549091.
- Warren K, Burden H, Abrams P. Mirabegron in overactive bladder patients: efficacy review and update on drug safety. Ther Adv Drug Saf. 2016 Oct;7(5):204-216. doi: 10.1177/2042098616659412. Epub 2016 Jul 19. PMID: 27695622; PMCID: PMC5014049.
- Wein AJ. Diagnosis and treatment of the overactive bladder. Urology. 2003 Nov;62(5 Suppl 2):20-7. doi: 10.1016/j.urology.2003.09.008. PMID: 14662403.
- QuickChange Men's Incontinence Wrap. (n.d.). QuickChange Men’s Maximum Absorbency Incontinence Wrap by UI Medical. QuickChange Men’s Incontinence Wrap. https://quickchange.com/?srsltid=AfmBOooEXy4jFfHUwUo7g4i4DLWak1BZUNCY7EhPgPpsCUHs3nySLEUJ